Compliance & Enforcement – Canada
Canadian consumers are exposed to a variety of health products in today’s market place and on-line. Often products originate from jurisdictions with a less stringent regulatory regime when compared to Canada’s regulations. The sale of unapproved health products may pose safety issues especially if they are sold or manufactured in contravention to Health Canada regulations. Given the ease of availability of so many products it has become increasingly important to have a strong and dynamic compliance and enforcement function to minimize risk and protect the health of Canadians at all times.
Health Canada has therefore made some updates to their compliance and enforcement program by establishing a new division, the Regulatory Operations and Regions Branch (RORB). The RORB is a dedicated compliance and enforcement branch within Health Canada overseeing the Compliance and Enforcement Policy framework to ensure that regulated parties maintain their responsibilities as per the Food & Drugs Act, the Natural Health Product Regulations and Cosmetic Regulations.
RORB’s mission is to lead compliance and enforcement activities and complementary scientific programs that will serve to inform and protect Canadians from health risks associated with products, substances and their environment. Through RORB, Health Canada administers the compliance program in three distinct stages which increase in severity, as described below.
Compliance promotion
At this early stage Health Canada will focus on helping regulated parties be aware of, access, understand and comply with the acts and regulations. Compliance promotion focuses on raising awareness and educating regulated parties about their obligations under the various acts and regulations. Health Canada publishes policies and guidance documents so that regulated parties understand Health Canada’s expectations for compliance.
Those parties that are interested in selling health products or cosmetics in Canada can refer to the relevant regulations in order to be confident they are providing products to Canadians that are imported, manufactured or distributed in compliance with Canadian regulations.
Compliance monitoring
Compliance monitoring includes targeted oversight of risk and efforts geared to a specific product that may be unsuitable for sale in Canada. RORB uses compliance monitoring to verify that products and regulated parties comply with the law. Compliance monitoring actions may include obtaining or analyzing information on products, regulated parties’ activities and processes or by carrying out inspections. Products may be analyzed at this stage to evaluate compliance and identify risks. Compliance monitoring may be triggered by complaints or adverse event reports filed sent to Health Canada.
Compliance Enforcement
In extreme situations of non-compliance enforcement may be used by Health Canada to bring a regulated party, product, or activity into compliance with the laws and therefore prevent future non-compliance.
Depending on the applicable acts and regulations, compliance enforcement at this high level may include the publication of safety alerts, advisories or other risk communications, the mandatory request to stop sale or initiate a product recall. Health Canada may also require changes to product labels in order to bring a product into compliance with Canadian regulations. In extreme non-compliance situations Health Canada may decide to prevent the sale, import, and advertisement of non-compliant products, cancel or suspend product licenses, seize products at retail or border locations, and impose financial penalties.
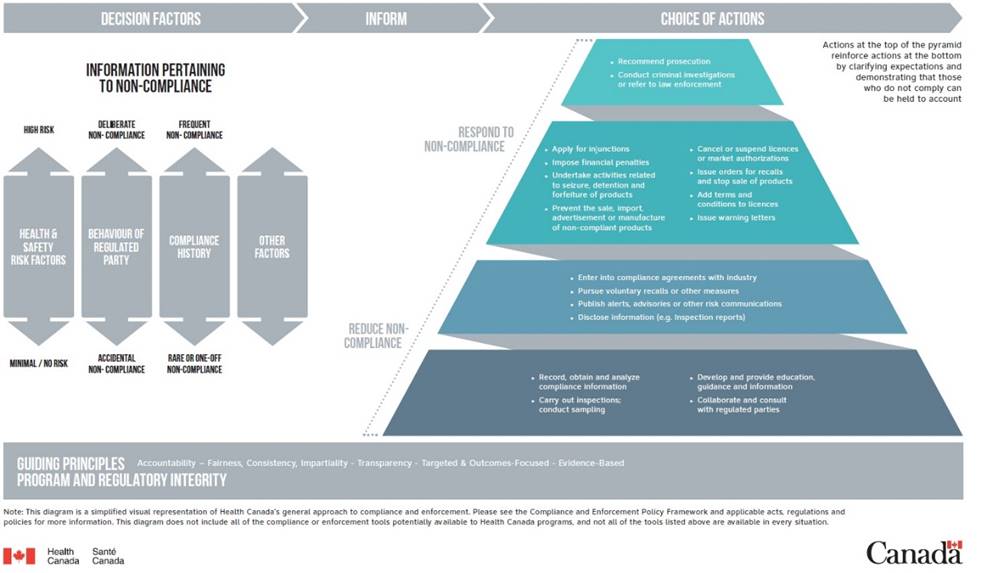
Health Canada’s general approach to compliance and enforcement can be viewed as a pyramid when it comes to actions and decision making. Actions at the top of the pyramid reinforce actions at the bottom by clarifying expectations and demonstrating that those who do not comply can be held accountable (1).
Health Canada’s Approach to Compliance and Enforcement